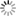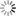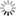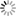Figg. 14-15: Chemical phenomena in Volta’s battery
“When we separate the elements of the piles which have been thus kept in action during several hours, or even days, under a cover which prevents the renewal of the atmospheric air, and having a constant communication kept up between their poles, we find that the metallic plates which compose them adhere to each other, and to the intermediate moistened cloths, with so great a force, that it is difficult to separate them. When this is done, we observe that the chemical action of the pile appears to have reacted on it, and produced remarkable alterations on its own elements. If the pile has been raised, according to the order, zinc, moisture, copper, zinc, &c., fig. 14, and placed on its zinc base, we observe invariably that particles detached from the inferior zinc plate have been carried to, and have fixed themselves on the plate of copper above it, while particles of copper have been transported to the superior zinc, and so on from the bottom to the top of the column. If the situation of the pile is the reverse, namely, copper, moisture, zinc, copper, &c., fig. 15, the copper descends upon the zinc, which is below it, and the zinc on the copper, from the bottom to the top of the column. The direction of the transport along the pile is reversed, but it remains the same relatively to the order of the elements of which the apparatus is composed” (p. 436).