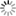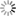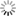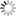“If we solder together two thin circular plates, the one of zinc and the other of copper, and if, after having laid this compound plate with the copper side on the hand, we cover the zinc side with a humid conductor, whose electromotive force is insensible, with a piece of cloth, for example, soaked in water, or some saline solution, all the conducting bodies which we place above this system will share in the excess of the vitreous electricity of the zinc side, and of the humid body which covers it. If then, on this first system, we place another similar one, so that its copper side may lie on the moistened cloth, this second system will then, as a conducting body, share the excess of the vitreous electricity of the first zinc side; and the second piece of zinc will, besides, take a new excess of electricity equally vitreous, produced by the electromotive force of the copper to which it is soldered. In thus adding successively several similar systems on each other, we obtain an apparatus in which the electric state of the successive pieces will go on augmenting from the bottom to the top, according to the number of pairs superimposed.
Such is the admirable instrument now universally known under the name of the Voltaic Pile, and by which both Natural Philosophy and Chemistry have obtained such astonishing results. […]
Take now a new piece of copper and zinc, similar to the first, and after having touched its copper side and insulated it, place this side upon the moistened cloth, as represented in fig. 7. According to Volta, two actions now begin: first, the zinc side of this second piece preserves the excess of vitreous electricity + 1, which it acquires from its contact with the copper; second, the whole system of the piece shares the free electricity of the cloth, as every other conducting body would do. The cloth renews this electricity, by drawing a supply from the inferior piece of zinc, this latter from the copper, and the copper from the ground; so that, after a certain time, which, if the conductibility be perfect, must be infinitely short, there arise a state of electrical equilibrium where the quantities of free electricity are such as is represented in the following table:
Superior piece Zinc side, Zg soldered to Cg +2
Copper side Cg, communicating with the moistened cloth +1
Inferior piece Zinc side, Z1 soldered to C1 +1
Copper side, C1, communicating with the ground 0” (p. 432)
Back to the table.