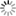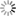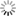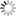Fig. 16: Experiment with Volta’s battery on the decomposition of water
“We have already seen […] the singular power which the Voltaic apparatus possess of separating the constituent principles of water. This experiment, a thousand times repeated, has been elaborately studied in its details, and has led to conclusions very useful in respect to other chemical decompositions. We shall, for this reason, therefore, first of all describe this process. The most convenient apparatus for doing it well, seems to be that which has been contrived by Messrs [Joseph Louis] Gay-Lussac and [Louis Jacques] Thénard, fig. 16. E E is a glass funnel, the mouth of which B is closed by a stopper coated sealing-wax, across which two wires of platinum are made to pass parallel, and distant from each other nearly half an inch; this wires rise within the funnel an inch and a half, or two inches, above the bottom of it. Water is then poured into the funnel, and each wire is covered by a small glass tube sealed in the top, and also filled with water. The external extremities of the wires are then made to communicate each of them with a pole of the pile, and the apparatus is arranged. After it has acted for some time, the communication between the two poles is interrupted, and measuring of the volume of the gas disengaged under each covered glass, we then find twice as great a volume of hydrogen as of oxygen. These are, in fact, the proportions with constitute water; for, on re-establishing the combination, there remains no gaseous residuum; at least, when the water exposed to the electrical current has been previously deprived of its air, and is preserved from the contact of this fluid during the operation, which may be done, either by covering the funnel with a cover properly luted, or in placing it in a vacuum. Without this precaution the gases disengaged by the pile would mix with portions of atmospheric air, either previously contained in the water, or absorbed by it during the operation; so that the nature and the proportion of the product would be altered by these circumstances. But, besides this, in order to lose nothing of the action of the pile, the communication of the decomposing wires with the extreme elements must be perfectly established; and nothing is more convenient for this purpose, than plunging them into a little cup of glass filled with mercury; in which are plunged two thick wires of iron, cemented to the extreme plates of the electromotive apparatus.
With this arrangement Messrs Gay-Lussac and Thénard have observed, that the quantity of gas disengaged in a given time by the same pile, whether constructed with moistened cloths or with troughs, varies considerably according to the nature of the substances dissolved in the water with which the funnel is filled. Concentrated saline solutions, and compounds of water and acids, give the most abundant and most rapid disengagement. This phenomenon diminishes as the proportions of salt or of acid become smaller; and lastly, when the funnel contains only boiled and perfectly pure water, almost no more gas is disengaged. Thus pure water, which transmits powerfully the electricity which is excited by our ordinary machines, becomes almost an insulating substance in the case of the weak repulsive forces to which the electromotive apparatus gives rise” (p. 439).